New Medical Device Guidelines
Medical Pharmaceutical Translations • Oct 7, 2019 12:00:00 AM
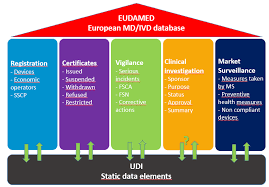
Medical device manufacturers take note! On September 26th, the European Commission published new guidance from the Medical Device Coordination Group about requirements for the Summaries of Safety and Clinical Performance (SSCP) for medical devices. This new guidance lists the requirements and recommendations for a device SSCP. The items address areas such as clinical evaluation incorporating both favorable and unfavorable data. It also states that documents must be written clearly and that differentiation needs to be made for physician versus patient-directed information.
Two pieces of the guidance are of specific interest to us, as a translation provider. First, the guidance recommends that lay and medical terms reside side-by-side in patient areas of an SSCP. This is an interesting concept for translation as languages handle “lay” medical explanations quite differently. It also impacts the way a document is written and constructed (prior to translation). Secondly, the SSCP has to be translated in to the accepted languages of each EU member state (in which the device will be marketed). While many device manufacturers hand translation off to people who are NOT experts in compliance and regulatory requirements, this guidance makes clear how important it is to have an expert partner who not only can accurately translate the technical components of an SSCP, but who can truly be a market-specific partner.
