The rise in decentralized clinical trials
Medical Pharmaceutical Translations • Sep 29, 2020 12:00:00 AM
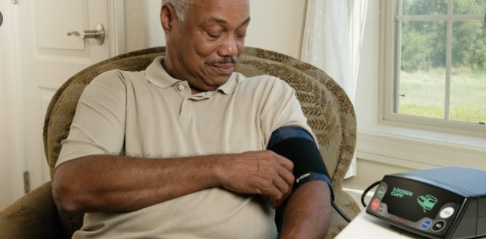
The coronavirus pandemic has changed so much about our world, including the way clinical trials are performed.
A new article suggests that this “new normal” may become a constant.
80% of non COVID-related clinical trials and research were interrupted or stopped entirely in the early days of the pandemic. But some were able to move forward by making an important and significant change: Instead of requiring trial participants to be monitored on-site, trials were decentralized, relying on home monitoring.
Interestingly, decentralized trials may outlast the pandemic, since they allow for greater flexibility in a number of ways. Of course, this won’t be possible for all trials, but the pharma industry may very well see a significant shift towards telehealth in yet another, less expected way.
Read on to learn more about the rise of decentralized clinical trials.
Contact Our Writer – Alysa Salzberg
